Michel Lièvre
Speeches:
-
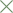
Meta analysis of 200mg vs 600mg
Michel Lièvre
Clinical Pharmacology Unit of Lyon, Faculté de Médecine Laënnec, Lyon, France
Although mifepristone has been approved in Europe, USA and Israel for termination of
pregnancy (TOP) at a dose of 600 mg in combination with prostaglandins, 200 mg is a
widely used dose, and has even been recommended by the WHO. We have therefore
assessed the evidence in favor of using 200 mg instead of 600 mg mifepristone for TOP.
Two main end points have been considered jointly: success (complete expulsion of the
conceptus) and ongoing pregnancy, the worst modality of failure. Because it is impossible
to prove the identity of two treatments, choosing between 200 mg and 600 mg
mifepristone has been dealt with as a non-inferiority issue. Non-inferiority limits have been
set from the pivotal studies used to grant the marketing authorization in France, resulting
in absolute values of -4% for success and 0.5% for ongoing pregnancy, corresponding to a
consented loss in success of 4% and a consented increase in ongoing pregnancies of
67%. The results of the 4 randomized trials comparing 200 mg with 600 mg mifepristone
(in combination with either oral misoprostol or intravaginal gemeprost) have been
summarized by a meta-analysis (rate difference method). These studies involved 1739
women allocated to 200 mg and 1743 to 600 mg mifepristone at up to 63 days
amenorrhea. Success ranged between 89.3% and 93.6% in the 200 mg and between
88.1% and 94.3% in the 600 mg group. Ongoing pregnancy ranged between 0.55% and
2.78% in the 200 mg and between 0% and 1.89% in the 600 mg group. The meta-analysis
showed a 0.4% [-1.4%, 2.3%] absolute difference in rate of success, allowing to conclude
to the non-inferiority of 200 mg compared with 600 mg mifepristone. For ongoingpregnancy, the difference was 0.4% [-0.3%, 1.0%], which did not allow to consider 200 mg
non-inferior to 600 mg mifepristone. Two sensitivity analyses gave similar results.
Conclusion. Although similar rates of success can be expected from 200 and 600 mg
mifepristone combined with either misoprostol or gemeprost, it cannot be excluded that the
use of 200 mg may lead to a clinically significant increase in the number of ongoing
pregnancies.
